Bisphosphonates block bone resorption. They have been widely used for years and although there can be side effects, the medical profession considers them generally safe and effective.
Most are taken orally, as a pill, either once a day or once a week. Bisphosphonates can be given intravenously; formulations are available for patients who cannot tolerate oral bisphosphonates. Some  patients cannot sit upright for 30 to 60 minutes which is a requirement for some drugs. The bisphosphonate zoledronate can be administered through a patch.
patients cannot sit upright for 30 to 60 minutes which is a requirement for some drugs. The bisphosphonate zoledronate can be administered through a patch.
Bisphosphonates are employed for:
Bisphosphonates bind strongly to the hydroxyapatite in the bone and interferes with osteoclasts, which are the bone cells that break down bone. The bisphosphonate molecules are incorporated into newly formed bone as they are analogs of pyrophosphate.
Hydroxyapatite is a calcium phosphorus compound that makes up about half of bone mass. Compared to other drugs, bisphosphonates stay in the body a very long time. Most drugs are excreted or metabolized by the body in a day or two. Bisphosphonates stay in the body for years through cycles of bone resorption and deposition.
This class of medical compounds all has a chemical structure that includes a P-C-P structure. Some bisphosphonate medicines are nitrogen compounds – their molecules include nitrogen atoms and they can be called aminobisphosphates. These include the most widely used bisphosphonates: risedronate, pamidronate, ibandronate, alendronate, and zoledronate. These nitrogen compound bisphosphonates more effectively slow boney material resorption to the blood than the simple bisphosphonates: etidronate, clodronate, and tiludronate. (There is evidence that nitrogen-containing bisphosphonates can reduce the risk of breast cancer.)
At a biochemical level, there are differences in how the two types (nitrogen-containing vs simple) work. This is pretty technical, but the simple drugs are metabolized by the osteoclasts and the resulting metabolites are essentially poisonous to the osteoclasts. The nitrogen-compound drugs disrupt protein prenylation, which moves to help detach the osteoclasts from the bone mass.
Market Share and Sales:
Over 10 million prescriptions for alendronate were written in the US in 2016. Merck's patent expired more than a decade ago. Generic alendronate from other manufacturers now domainates the market.
Dosage and Forms:
10mg and 70mg tablet
Generic formulations manufactured by:
Approved Indications for use:
Non-approved but known (off label) uses
Dosage and Forms:
Approved Indications
Non-approved but known (off label) uses
The patent expired in 2011 and generic forms are now available.
Dosage and Forms:
5mg, 35mg, 75mg and 150mg tablet
Approved Indications for use:
Non-approved but known (off label) uses
Dosage and Forms:
Blister Package containing one (1) 35mg Actonel and six (6) 1250mg calcium
carbonate tablets
o Actonel taken on day 1 and calcium taken on days 2 - 7
Approved Indications for use:
Non-approved but known (off label) uses
Market Share and Sales:
Dosage and Forms:
Approved Indications for use:
Non-approved but known (off label) uses
The patent for ibandronte expired and the FDA approved generic versions in 2012.
Dosage and Forms:
Approved Indications for use:
Non-approved but known (off label) uses
Dosage and Forms:
Approved indications for use:
Non-approved but known (off label) uses
The UK's National Osteoporosis Guideline Group (NOGG) recommends stopping treatment after three years. Research has shown the benefits persist three years after the medicine is stopped, at which time the physician is recommended to take another assessment of the patient for possible restart of therapy.
Dosage and Forms:
generic formulations manufactured by:
Approved Indications for use:
Non-approved but known (off label) uses
Dosage and Forms:
Injectable (IV) available in 30mg and 90mg vials
Generic formulations manufactured by: Bedford labs
Approved Indications for use:
Non-approved but known (off label) uses
Patent expired in 2009 but little chance for generic manufacturing due to limited use.
Dosage and forms
240mg tablet equivalent to 200mg tiludronic acid
Approved Indications for use:
Non-approved but known (off label) uses
Binosto is alendronate, but delivered in a different form. It is an effervescent tablet that the patient takes once a week. The manufacturer hopes that if it goes down easier than the pill form and will be attractive for that reason.
From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5768298/
| Bisphosphonate | Prophylactic Dose | Treatment Dose |
|---|---|---|
| Alendronate | 5 mg once daily or 35 mg once weekly | 10 mg once daily or 70 mg once weekly |
| Risedronate | 5 mg once daily or 35 mg once weekly | 5 mg once daily or 35 mg once weekly or 150 mg once monthly |
| Zoledronic acid | 5 mg IV every 2 years | 5 mg IV once yearly |
| Ibandronate | 2.5 mg once daily or 150 mg once monthly | 2.5 mg once daily or 150 mg once monthly or 3 mg IV every 3 months |
Bisphosphonates were at the center of legal trouble because of a complication known as "Dead Jaw Syndrome" or Osteonecrosis of the Jaw. The lawsuits were primarily targeted against the makers of Fosamax but all bisphosphonates may potentially cause this disease.
Bisphosphonates are often called bone builders, but some feel a more apt description of their action is in hardening bone, and consequently making bone tissue more btittle after long-term use.
These drugs may cause or exacerbate upper gastrointestinal disease such as esophagitis and flatulence or abdominal pain. Intravenous bisphosphonates pose a risk of kidney damage, which is why patients taking them are monitored for creatinine in the bloodstream.
Osteonecrosis of the jaw is also a rare but serious side effect.
A study found evidence that bisphosphontes increase the risk of thigh fracture. The increased risk, although small, gets higher the longer the patient takes the medication. The problem appears to be that the some bone resorption is important in healthy bone physiology, and that the bisphosphonates are too effective in stopping turnover.
The FDA issued an advisory that doctors should reassess the use of these drugs, given concerns about side effects and scant evidence that the drugs help people without osteoporosis. The FDA requires bisphosphonates be labeled "the optimal duration of use has not been determined."
Here is a situation where the doctor must formulate a strategy. Younger patients and patients considered at lower risk for fractures, may be better candidates for discontinuing bisphosphonate therapy after a few years.
Some experts are questioning the use of bisphosphonates for patients with osteopenia. And osteoporosis patients on bisphosphonates might consider discussing a "drug holiday" with their doctors, during which they would stop taking the drug for a while. The FDA has said that most women could stop taking bisphosphonates after 5 years, as the marginal benefit from continued use was minimal. A study published in 2014 concluded patients who had taken alendronate for three years could safely take a one year holiday with no increase in fracture risk. It is widely known among scientists that the pharmacologic effects of these drugs persist long after patients stop taking the medication.
Clearly despite side effects and adverse event potential, this class of drug is beneficial to many and has been a major tool in the treatment of bone disorders. After rapid growth, the use of bisphosphonates appears to have peaked in the years before 2010. One report stated the use dropped 50% between 2008 and 2012. 50% sounds like a lot, but it is understandable there would be a drop, due to increases in concerns about side effects, a reduction in drug company support following expiration of patents, and worries about long-term or indefinite use and the rise in awareness of the possibility of drug holidays and research showing stopping the drug after a period of years does not seem to result in an increase in facture risk.
Related: Risks and benefits of long-term bisphosphonate therapy.
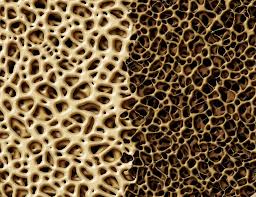
Bisphosphonates block bone resorption. They have been widely used for years and although there can be side effects, the medical profession considers them generally safe and effective. More
Selective Estrogen Receptor Modulators (SERMs) are chemical compounds that act on the estrogen receptors in the body. More.
