Roughly speaking, there is no difference. They are the same active chemical, C4H12NNaO7P2. They may have different filler materials and may be packaged differently, but the Food and Drug Administration requires all generic equivalents to be as effective as their branded counterparts. The drug company Merck owns the brand name Fosamax® and only they and their representatives can legally sell alendronate under that name in the United States.
A brand name drug is considered an innovator, or discovery, drug because it has an original composition. Because of this new composition, the drug has three overall phases of testing: laboratory, animal, and human. The research company must first file a New Drug Application or (NDA)1 with the FDA Center for Drug Evaluation and Research (CDER). They must provide evidence regarding the risks and benefits of testing the compound based on laboratory/animal testing results. In order to test human subjects, the pharmaceutical company must provide evidence that the benefits of the potential drug treatment outweigh the risks of adverse events in humans. Once this is approved, a series of clinical trials is done. Evidence of the drug's effectiveness, reports of adverse events, study results, and analyses are sent to the CDER with added sections to their NDA, which includes multiple detailed sections on everything from chemical composition to packaging. This process, from research to clinical trials, can be very expensive. Because of the time and expense, the company can patent their drug composition with the idea of recouping some of the costs involved from the long research and approval process. This patent lasts 20 years from the date of the first US patent application.
Companies authorized by the FDA to make generic alendronate include Sandoz, Barr, Teva, and Ivax.
Doctors, nurses, and the larger medical industry as well as consumers are familiar with the word Fosamax® and typically refer to alendronate as Fosamax. You might hear someone mention they are taking generic Fosamax® or even have your doctor tell you he is giving you a prescription for Fosamax® even if the pharmacy substitutes a generic.
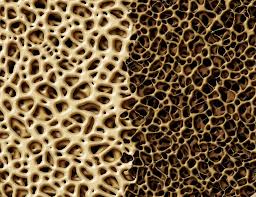
Bisphosphonates block bone resorption. They have been widely used for years and although there can be side effects, the medical profession considers them generally safe and effective. More
Selective Estrogen Receptor Modulators (SERMs) are chemical compounds that act on the estrogen receptors in the body. More.
